Various Corrosion Types with Classic Photographs
Crevice Corrosion of Stainless Steel - Crevice  corrosion is the localize corrosive attack that occurs
as a result of the occluded cell that forms under a crevice on the metal
surface. On this stainless steel test coupon from an ASTM G48 Method B ferric
chloride test, the crevice was a non-metallic block. Note that the test coupon
was not attacked by corrosion except in the middle of the coupon where it was
in contact with the block and on the edges were a rubber band used to hold the
block in place also came in contact with the metal surface.
corrosion is the localize corrosive attack that occurs
as a result of the occluded cell that forms under a crevice on the metal
surface. On this stainless steel test coupon from an ASTM G48 Method B ferric
chloride test, the crevice was a non-metallic block. Note that the test coupon
was not attacked by corrosion except in the middle of the coupon where it was
in contact with the block and on the edges were a rubber band used to hold the
block in place also came in contact with the metal surface.
 Microbiologically Induced Corrosion in a Sour Gas
Pipeline - The highly localized corrosion
shown in the figure is typical of that resulting from microbial action. One of
the features of this type of attack are the elongated pits which tunnel into
the specimen often in an irregular manner. The pit was one of several located
near the gas/water interface. The pipeline was left for a prolonged period in a
shut-in (static) condition which promoted the growth of bacteria and highly
localized corrosive attack. Sulfate reducing bacteria were suspected due to the
combination of sulfate species in the water and anaerobic conditions. The
corrosion was mitigated by a closer control of operating conditions and
chemical treatment.
Microbiologically Induced Corrosion in a Sour Gas
Pipeline - The highly localized corrosion
shown in the figure is typical of that resulting from microbial action. One of
the features of this type of attack are the elongated pits which tunnel into
the specimen often in an irregular manner. The pit was one of several located
near the gas/water interface. The pipeline was left for a prolonged period in a
shut-in (static) condition which promoted the growth of bacteria and highly
localized corrosive attack. Sulfate reducing bacteria were suspected due to the
combination of sulfate species in the water and anaerobic conditions. The
corrosion was mitigated by a closer control of operating conditions and
chemical treatment.
 Pitting in Aluminum - The localized pitting corrosionwas produced in aluminum floats on a
storage tank roof. The exposure conditions involved hydrocarbon fluids
following an initial hydrotest. The pitting occurred in the absence of
chlorides at a near neutral pH where aluminum would be expected to exhibit good
resistance to corrosion. Sulfur corrosion products were found in the pits and
sulfate reducing bacteria were suspected resulting from prolonged exposure to
hydrotest water.
Pitting in Aluminum - The localized pitting corrosionwas produced in aluminum floats on a
storage tank roof. The exposure conditions involved hydrocarbon fluids
following an initial hydrotest. The pitting occurred in the absence of
chlorides at a near neutral pH where aluminum would be expected to exhibit good
resistance to corrosion. Sulfur corrosion products were found in the pits and
sulfate reducing bacteria were suspected resulting from prolonged exposure to
hydrotest water.
Mesa Corrosion of Steel Tubing - Mesa corrosion is  one of the common types of corrosion experienced in
service involving exposure of carbon or low alloy steels to flowing wet carbon
dioxide conditions at slightly elevated temperatures. An iron carbonate surface
scale will often form in this type of environment which can be protective
rendering a very low corrosion. However, under the surface shear forces
produced by flowing media, this scale can become damaged or removed and
exposure fresh metal to corrosion. This localized attack produces mesa-like
features by corroding away the active regions and leaving the passive regions
relatively free of corrosion resulting the surface profile reminiscent of the
mesas produced in rock by wind and water erosion.
one of the common types of corrosion experienced in
service involving exposure of carbon or low alloy steels to flowing wet carbon
dioxide conditions at slightly elevated temperatures. An iron carbonate surface
scale will often form in this type of environment which can be protective
rendering a very low corrosion. However, under the surface shear forces
produced by flowing media, this scale can become damaged or removed and
exposure fresh metal to corrosion. This localized attack produces mesa-like
features by corroding away the active regions and leaving the passive regions
relatively free of corrosion resulting the surface profile reminiscent of the
mesas produced in rock by wind and water erosion.
 Velocity Accelerated Corrosion of Cupro-Nickel Tubing
- The corrosion features on the inside of
this heat exchanger tube were produced by high temperature, flowing seawater.
The alloy was 90-10 cupro-nickel which has limited flow resistance in seawater
of around 9 to 12 ft/sec (3 to 4 m/sec) depending on many factors. In this
case, the flow rate was in this range which produced the accelerated corrosion
on the outside portion of the bend. “Horseshoe” or “U” features are characteristic
of this type of attack which are oriented with the closed end of the features
pointing in the direction of flow.
Velocity Accelerated Corrosion of Cupro-Nickel Tubing
- The corrosion features on the inside of
this heat exchanger tube were produced by high temperature, flowing seawater.
The alloy was 90-10 cupro-nickel which has limited flow resistance in seawater
of around 9 to 12 ft/sec (3 to 4 m/sec) depending on many factors. In this
case, the flow rate was in this range which produced the accelerated corrosion
on the outside portion of the bend. “Horseshoe” or “U” features are characteristic
of this type of attack which are oriented with the closed end of the features
pointing in the direction of flow.
 Stress Corrosion Cracking of Stainless Steel - The example shown indicates many intersecting,
branched cracks with a transgranular propagation mode. These are typical of
stress corrosion cracking (SCC) in austenitic stainless steel. In this case,
however, the alloy was reported to be resistant to SCC in the NaCl brine
service environment. The location of cracking was limited to a region covered
by an elastomeric sleeve. Under the sleeve, evidence of severe general and
pitting corrosion were found and evidence of sulfur-containing corrosion
products. Analysis of the elastomer indicate that it was not the correct grade
and chemical degradation had occurred in service to produce organic acids and
sulfur compounds. This local environment resulted in enhanced localized
susceptibility of the material to pitting corrosion and SCC.
Stress Corrosion Cracking of Stainless Steel - The example shown indicates many intersecting,
branched cracks with a transgranular propagation mode. These are typical of
stress corrosion cracking (SCC) in austenitic stainless steel. In this case,
however, the alloy was reported to be resistant to SCC in the NaCl brine
service environment. The location of cracking was limited to a region covered
by an elastomeric sleeve. Under the sleeve, evidence of severe general and
pitting corrosion were found and evidence of sulfur-containing corrosion
products. Analysis of the elastomer indicate that it was not the correct grade
and chemical degradation had occurred in service to produce organic acids and
sulfur compounds. This local environment resulted in enhanced localized
susceptibility of the material to pitting corrosion and SCC.
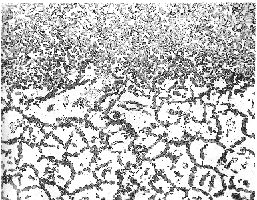 Carburization of Modified HK-40 Furnace Tubing - Massive carburization occurred in service due to upset
conditions in an ethylene furnace. Conditions that caused the failure included
excessive temperature in combination with high carbon activity in the process.
The attack resulted in the formation extensive surface carburization and severe
grain boundary carbides which progressed through the material from the I.D.
surface. These changes in microstructure resulted in severe embrittlement of
the tubing and premature failure.
Carburization of Modified HK-40 Furnace Tubing - Massive carburization occurred in service due to upset
conditions in an ethylene furnace. Conditions that caused the failure included
excessive temperature in combination with high carbon activity in the process.
The attack resulted in the formation extensive surface carburization and severe
grain boundary carbides which progressed through the material from the I.D.
surface. These changes in microstructure resulted in severe embrittlement of
the tubing and premature failure.
 Internal Coating Failure in a Pipeline - The I.D. coating failure was characterized by the
formation of blisters in a field applied epoxy coating. Upon closer
examination, the blisters were found to formed within the coating resin as a
result of foaming during the coating operation. This is contrasted from the
more common case where blistering is initiated on the metal surface by
contamination or inadequate preparation. In this case, water was adsorbed from
the I.D. environment by the epoxy resin and collected in the voids within the
epoxy resin resulting in the formation of blisters within the coating.
Internal Coating Failure in a Pipeline - The I.D. coating failure was characterized by the
formation of blisters in a field applied epoxy coating. Upon closer
examination, the blisters were found to formed within the coating resin as a
result of foaming during the coating operation. This is contrasted from the
more common case where blistering is initiated on the metal surface by
contamination or inadequate preparation. In this case, water was adsorbed from
the I.D. environment by the epoxy resin and collected in the voids within the
epoxy resin resulting in the formation of blisters within the coating.
 Oxidative Degradation of Carbon-Carbon Composites - Carbon-carbon fiber composites are advanced
materials with excellent strength to weight performance characteristics.
However, at high temperature in the presence of aerated atmospheres, the
carbonaceous fiber material will be susceptible to oxidation resulting in both
mass loss and a reduction in strength. Rapid thermal flucuations common in thin
components can work synergistically with the environmental degradation to
produce accelerated attack. Various glasseous, multilayer ceramic coatings are
often used to provide resistance to this form of environmental attack which
must have both chemical resistance and controlled thermal expansion properties.
Oxidative Degradation of Carbon-Carbon Composites - Carbon-carbon fiber composites are advanced
materials with excellent strength to weight performance characteristics.
However, at high temperature in the presence of aerated atmospheres, the
carbonaceous fiber material will be susceptible to oxidation resulting in both
mass loss and a reduction in strength. Rapid thermal flucuations common in thin
components can work synergistically with the environmental degradation to
produce accelerated attack. Various glasseous, multilayer ceramic coatings are
often used to provide resistance to this form of environmental attack which
must have both chemical resistance and controlled thermal expansion properties.
 Localized Corrosion in 13Cr Stainless Steel - In many industrial process environments, stainless
steels provide for long term serviceability by maintaining a passive oxide layer
on the metal service. The sample in the figure shows signs of local area
corrosion from loss of passivity in an aqueous brine containing dissolved
carbon dioxide and hydrogen sulfide. The passive regions still show the signs
of the original machining marks whereas the active regions show a black
corrosion product and extensive pitting. In environments with little or no
hydrogen sulfide, this material would be expected to exhibit excellent
corrosion resistance. However, the localized breakdown in the protective
passive layer in this case is the direct result of relatively high hydrogen
sulfide and chloride concentrations in the simulated service environment.
Localized Corrosion in 13Cr Stainless Steel - In many industrial process environments, stainless
steels provide for long term serviceability by maintaining a passive oxide layer
on the metal service. The sample in the figure shows signs of local area
corrosion from loss of passivity in an aqueous brine containing dissolved
carbon dioxide and hydrogen sulfide. The passive regions still show the signs
of the original machining marks whereas the active regions show a black
corrosion product and extensive pitting. In environments with little or no
hydrogen sulfide, this material would be expected to exhibit excellent
corrosion resistance. However, the localized breakdown in the protective
passive layer in this case is the direct result of relatively high hydrogen
sulfide and chloride concentrations in the simulated service environment.